News
The Rise of Open Source in Clinical Research
How Much Money Can Be Saved by Using Centralized Monitoring in Clinical Trials?
Validating Centralized Monitoring Tools
The tools that sponsors use in clinical research must be validated to ensure they work as intended. Regulators refer to the FDA's Code of Federal Regulation Title 21 Part 11 for the validation of electronic data capture (EDC) systems, the International Society for Pharmaceutical Engineering's (ISPE) Good Automated Manufacturing Practice (GAMP) for the validation of systems used in the manufacturing of medicinal products and the Parenteral Drug Association's (PDA) Technical Report 80 for the validation of laboratory equipment. However, centralized monitoring tools that clinical operations use to make quality-related decisions are not explicitly covered in any of these documents. How can...
Performing risk assessments for the risk-based monitoring of clinical trials
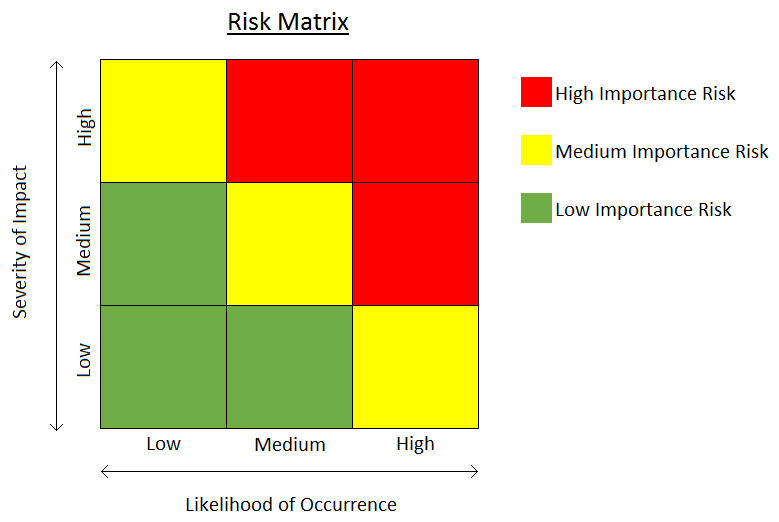
Risk assessment is an integral part of risk management. In clinical trials, it serves to lay the foundation of risk-based monitoring processes. Regulators currently encourage its use to improve the safety and the quality of clinical trials. In practice, assessing risks is difficult but clinical research professionals must become acquainted with the concept of risk assessment in order to reap the full potential of risk-based monitoring. Regulatory Perspective The FDA’s most recent guidance is fairly specific about the importance of conducting a risk assessment to identify risk and develop a monitoring plan: The risk assessment serves to identify and understand...
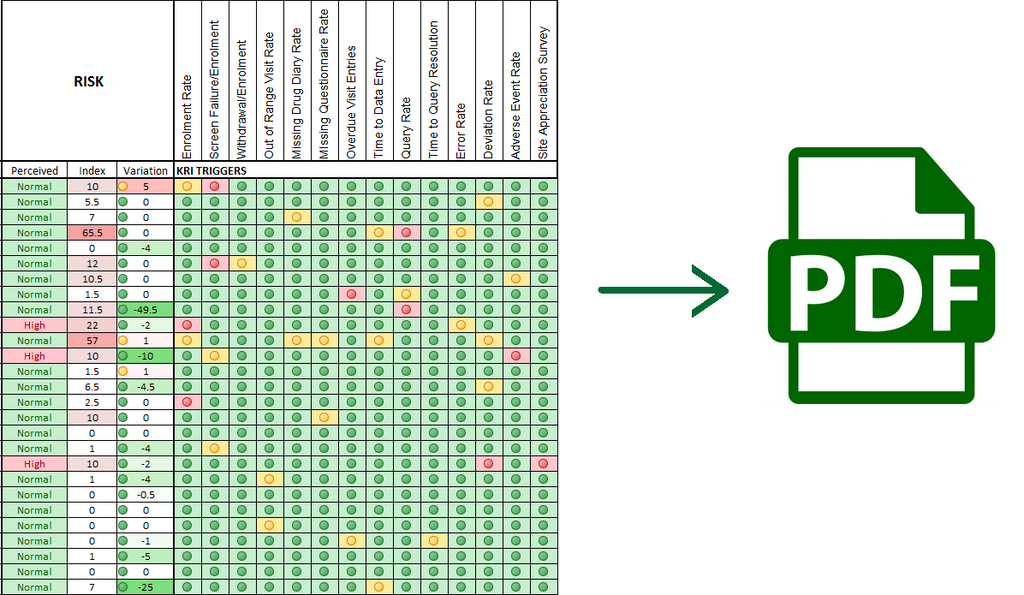
What's In a Centralized Monitoring Report
The centralized monitoring report is the best channel to share risk signals with clinical trials stakeholders. In addition to being the perfect tool for the review of site-specific Key Risk Indicators (KRI) metrics with clinical operations teams, producing periodic reports ensures the traceability of the centralized monitoring activities for regulatory audit purpose. Reports are also essential for the continuous improvement of centralized monitoring processes. The TransCelerate BioPharma Initiative specifically states that Monitoring reports should serve as tools for the team members to communicate a concise, high-level summary of monitoring activities, issues and associated actions. It’s imperative that companies build off...