News
Setting Limits in Centralized Monitoring
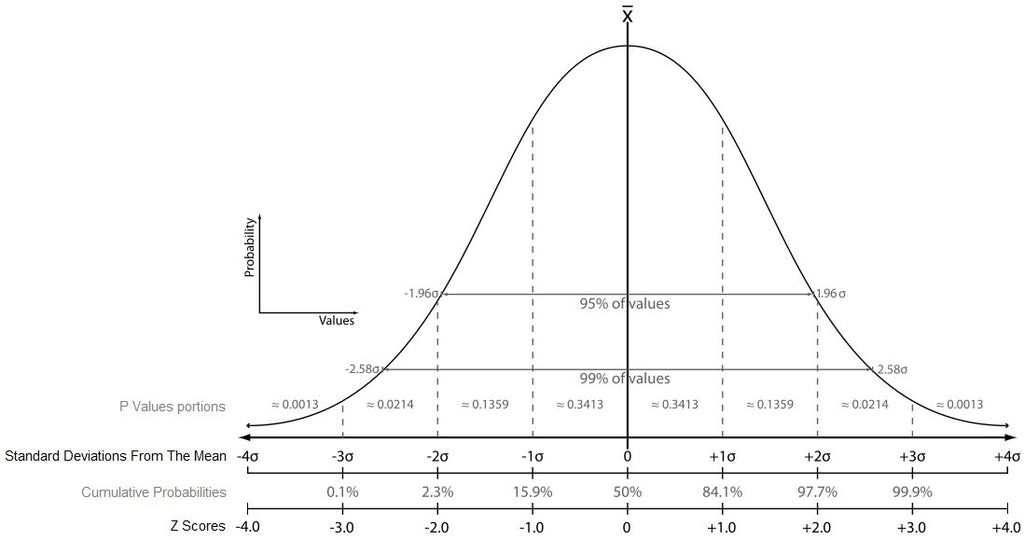
When using a centralized monitoring approach, Key Risk Indicators (KRIs) metrics are calculated for individual study sites and compared against limits, also known as “thresholds” or “tolerance level”, beyond which sites may be considered abnormal and warrant investigation. Limit values must be carefully chosen to be able to detect risk without triggering too many false signals. Information relevant to the choice of limits can be obtained by executing a trial’s risk assessment and by calculating site-specific KRIs statistical distributions. Below are some points to consider when choosing limits. Limits Based on Risk Assessment Risk identified through the risk assessment should...
Planning for Change in Centralized Monitoring
Writing an Integrated Quality and Risk Management Plan (IQRMP) involves identifying risk factors and developing a contingency plan to manage the risks that cannot be eliminated. Risk factors can be monitored using Key Risk Indicators (KRI) which are metrics calculated from the collected data associated with risk factors. For instance, most studies consider the AE rate a risk factor and will monitor it on a periodic basis by calculating the site-specific number of adverse events divided by the number of subject-days. A risk signal is typically triggered when a Key Risk Indicator (KRI) metric’s value falls beyond its set limits...
Centralized Monitoring False Risk Signals
Performing clinical trial Centralized Monitoring (CM) involves evaluating risk signals such as site-specific Key Risk Indicators (KRI) metrics that fall outside set limits and monitoring their progress. Many systems use dashboards with traffic lights-type risk signals to highlight sites with computed KRI metrics values that fall outside their normal range. When these lights are triggered, they usually initiate a sequence of actions by the clinical team aimed at mitigating the risk they represent. Nevertheless, upon closer examination, it appears that most risk signals are the result of perfectly normal phenomena and actually requires no action by the clinical team. Here...
The Basics of Centralized Monitoring
In the context of multicenter clinical research, Centralized Monitoring (CM) is the most efficient way to ensure subject’s safety, trial integrity and data quality. As it permits the study team to proactively detect anomalous data trends, CM improves the quality of the regulatory submissions with a direct impact on the time to marketing approval. Since publication of the regulatory guidance on Risk-Based Monitoring (RBM) five years ago, the concept of CM has developed amid the emergence of technological enablers that make clinical research more data-driven than ever. Today, regulators encourage the use of CM in conjunction with on-site monitoring to...
Clinical Trial Risk-Based Monitoring with Excel
Current regulatory and economic incentives are prompting clinical research organizations to integrate risk management strategies into clinical operations and to adopt technological enablers for the efficient monitoring of clinical trials. Today’s drug discovery is meeting difficulties as R&D investments are being restricted and an ever-longer and more costly drug development process faces the clinical trial enterprise. Approximately two-thirds of the total drug R&D costs are associated with clinical phases of development, of which 70% are allocated to Phase II/III activities. The traditional process of sponsor oversight over clinical trials is currently being challenged as research suggests that certain monitoring practices...